Complex mixtures: H2O-NaCl-CO2
Introduction
Natural fluids can consist of complex mixtures of saline water and gas(es).
The H2O-NaCl-CO2 system is representative of numerous metamorphic fluids and can be considered a model system for complex water-salt-gas(es) mixtures.
The carbonic nature of inclusion is undoubtedly recognized by the observation of three phases at room temperature: aqueous liquid, carbonic liquid and carbonic vapour.
Video
The inclusion used in this video was chosen in an alpine crack of the Tessin region (Switzerland). Numerous large inclusions are easily found in the sample as primary inclusions. They are typical three-phase.
Video description
The choosen inclusion is typically three-phase at room temperature.
It contains an aqueous liquid phase, a carbonic liquid phase (external bubble) and a carbonic vapour bubble (inner bubble).
During cooling three solidification processes can be observed including a subtle contraction of the bubbles.
The first solidification occurs at about -32.2°C. The meniscus of the carbonic liquid deforms suddenly. This is the temperature where a hydrate composed of water and CO2 molecules crystallizes. This phase is called clathrate.
The second solidification (-40.9°C) also includes a granulation of the liquid phase. This phase transition corresponds to the formation of ice.
Going on cooling a third solidification occurs inside the bubbles around -94.9°C. It is associated with the solidification of liquid CO2 into solid CO2.
The heating sequence shows a sudden melting at -56.6°C; This is the melting of solid CO2 into liquid CO2.
The next phase transition is theoretically the melting of ice, not shown in the present sequence.
Going on heating the sequence presents the melting of clathrate. Clathrate melting is not easily observable as the refraction index of clathrate is similar to that of liquid water. The only way to observe clathrate is to apply a cycling method. During cycling the temperature is simultaneously increased and decreased. Heating will induce the progressive melting of clathrate and the subsequent release of the vapour bubble and its rounding; cooling will induce the growing of the clathrate crystals and the deformation of the liquid-vapour meniscus. Applying the cycling procedure allows to measure the clathrate melting temperature at 6.8°C.
Further heating leads up to partial homogenization of the carbonic sub-system: At a temperature of 31.3°C the inner and the intermediate bubbles homogenize by progressive disappearance of the meniscus (sub-critical homogenization).
Going on heating the inclusion will induce bulk homogenization (not shown in the video).
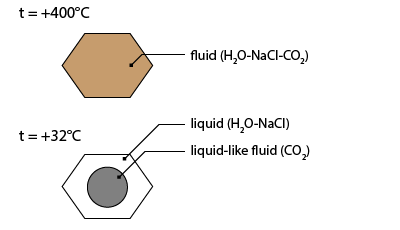
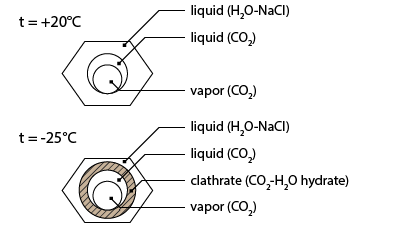
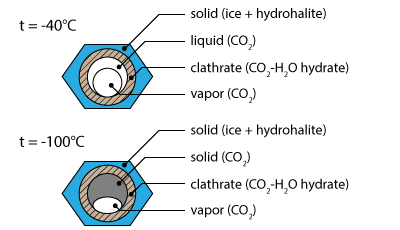
Interpretation
Interpretation of the sequence requires the use of complex phase diagrams for the 3-component system H2O-NaCl-CO2 (Dubois, 1992[1]).
The sequence presents 5 phase transitions during heating:
CO2 melting temperature: This temperature is at -56.6°C when CO2 is pure
ice melting temperature: This temperature is generally difficult to measure because a large quantity of water is consumed during the formation of clathrate
clathrate melting temperature: This phase transition corresponds to the decomposition of the clathrate:
CO2 • 5.75 H2O (clathrate) → CO2 + H2O
The clathrate melting temperature (in presence of CO2 vapour and CO2 liquid) is a function of salinity according to the following mathematical expression (Bozzo et al., 1973, Collins, 1979):
wt%NaCl = 15.52022 - 1.02342 x t - 0.05286 x t2
where t is the clathrate melting temperature expressed in °C
homogenization of the carbonic sub-system: The CO2 homogenization temperature can be used to determined the density of CO2 at homogenization [see page dedicated to the pure CO2 system]. After partial homogenization CO2 is in the fluid phase (but is vapour relative to the aqueous phase ... not so simple !!!)
bulk homogenization: This is the disapperance of the vapour phase or the liquid phase at high temperature (see page dedicated to the bulk homogenization)
More
Three-phase inclusions are very classical in metamorphic environments.
In addition, high density H2O-CO2 inclusions are characteristic of lode gold deposits (the so-called mesothermal) ( Groves et al., 2003[2]; Goldfarb et al., 2005[3]; Béziat et al., 2008[4])