Homogenization into the critical phase
Introduction
Whereas bulk homogenization generally occurs into the liquid phase (see previous page) or can occur into the vapour phase, a particular case is represented by homogenization into the critical phase.
In the case of critical homogenization the densites of each phase (liquid and vapour) become closer to each other during heating.
Homogenization therefore occurs by fading of the meniscus between the two phases.
Video
The video presented in this section corresponds to a fluid inclusion composed of pure water with the critical density.
This is a synthetic fluid inclusion.
Video description
At room temperature the inclusion is two-phase (liquid+vapour).
The inclusion is progressively heated. No change does occur during heating.
At about 360°C the meniscus between the two phases become very thin. The volume proportion of each phase did not significantly change up to this temperature.
At 374.1°C the meniscus between both phases completely disappears by complete fading. The density of each becomes identical.
During cooling of the inclusion the meniscus reappears rapidly.
Interpretation
The interpretation of the sequence can be made in the system H2O (pure water).
Two diagrams can be used, a temperature-pressure diagram and a density temperature.
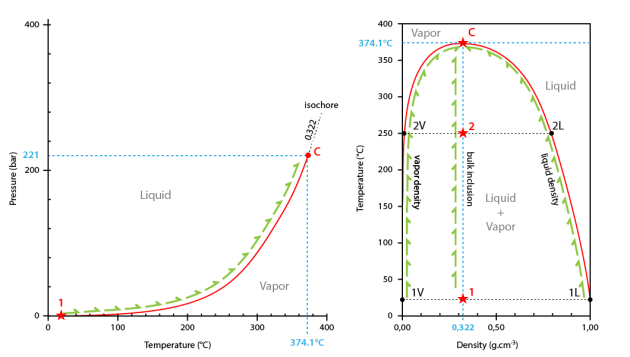
In the temperature-pressure diagram, the path followed by the inclusion starts at room temperature and on the liquid+vapour curve (point #1).
During heating, the inclusion follows the liquid+vapour curve to the critical point (374.1°C and 221 bar; point C).
The critical point is the extremity of the liquid+vapour curve that extends from the triple point (point T) to the critical point (point C) (boiling curve).
Critical homogenization occurs at 374.1°C.
Going on heating the inclusion, the path is now the critical isochore corresponding to the critical density (0.322 g.cm3).
In the density-temperature projection, the path followed by the inclusion is a vertical line corresponding to critical density (system at constant density) (0.322 g.cm3).
At the starting temperature (room temperature), the point corresponding to the whole inclusion lies inside the liquid+vapour field (point #1).
The density of the liquid phase is dl, and the density of the vapour phase is dv. During heating the density of the liquid phase decreases along the solvus (path from point #1L to point C), whereas the density of the vapour phase increase (path from point #1V to point C).
At 374.1°C both densities are equal. The meniscus disappears. Critical homogenization has occurred. The inclusion content if now fluid.
More
Critical homogenization is a rather rare phase transition observed in nature. It would correspond to inclusions trapped along the critical isochore, that is statistically unlikely.
Synthetic fluid inclusions with pure water with critical density are classical standards for calibrating microthermometric stages at high temperature (H standard 374.1°C).