Homogenization into the liquid phase
Introduction
Homogenization of an initially two-phase (liquid+vapour) inclusion into the liquid phase is probably one of the most common phase transition measured in fluid inclusions.
This corresponds to the disappearance of the vapour phase, generally following a decrease of the vapour phase size during heating.
Video
The video presents the homogenization of a complex fluid inclusion in a pegmatite of the Velay massif (French Massif Central; Dubois, 1992). The inclusion has a negative crystal shape (hexagonal) and presents a vapour percentage of about 60%.
Video description
At room temperature the inclusion is two-phase (liquid+vapour).
During heating the vapour proportion significantly decreases. At about 360°C the vapour bubble is now very small and starts moving rapidly. The rapid moving of the bubble is called the Brownian motion.
At 364.2°C the vapour phase now reduced to a point completely disappears. This is homogenization by vapour disappearance (i.e. into the liquid phase). The corresponding temperature is called the homogenizaton temperature, generally referred as Th.
Interpretation
The interpretation is possible in the temperature-pressure diagram. As the exact composition of the system is not exactly known in our case we will use a generic diagram.
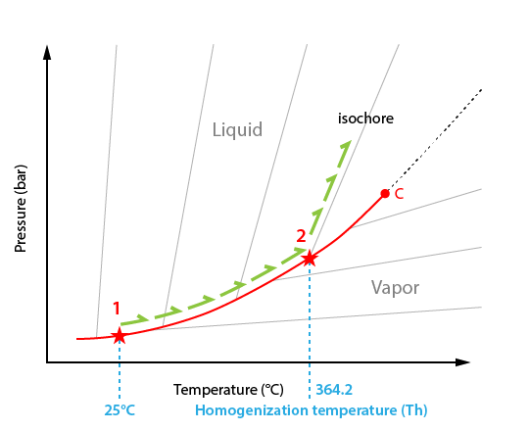
In starting conditions at room temperature the inclusion lies on the liquid-vapour curve (point #1).
During heating the inclusion follows the liquid-vapour curve. The only visible change is the regular reduction of the vapour bubble volume.
At the homogenization temperature (Th), the vapour disappears.
The path therefore leaves the liquid-vapour curve (point #2) and starts climbing up the isochore corresponding to the bulk inclusion density. An isochore is a curve of constant density.
More
Homogenization can be observed in quasi all types of inclusions as soon as they contain two phases at the starting temperature.
As slopes of isochores are systematically positive homogenisation temperature is an indicator of trapping conditions. Indeed the general relation can be written in all cases, whatever the chemical system:
Homogenization temperature ≤ trapping temperature
When the chemical system is known the bulk density of the inclusion can be estimated using the suitable phase diagram or an equation of state.